

| WHAT IS RADIOACTIVITY? | Order your RS-500 now! |
Atoms are not all stable. The excess energy contained in an unstable atom is released in one of a few basic particles and energetic waves. The Greek alphabet is used to name the particles (in the order of their discovery).
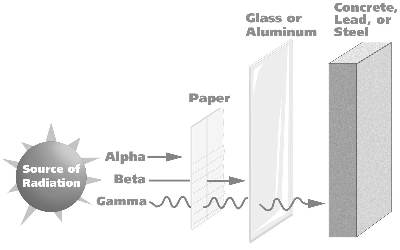
ALPHA PARTICLES
The alpha particle is the heaviest. It is produced when the heaviest elements decay. Alpha and beta rays are not waves. They are high-energy particles that are expelled from unstable nuclei. In the case of alpha radiation, the energy The particles leave the nucleus . The alpha particle is an helium atom and contains two neutrons and two protons. It leaves the nucleus of an unstable atom at a speed of 16,000 kilometres per second, around a tenth the speed of light. The alpha particles is relatively large and heavy. As a result, alpha rays are not very penetrating and are easily absorbed. A sheet of paper or a 3-cm layer of air is sufficient to stop them. Its energy is transferred within a short distance to the surrounding media. However, its short flight knocks about 450,000 electrons out of the surrounding atoms. The alpha particle emitter will not penetrate the outer layer of our skin, but is dangerous if inhaled or swallowed. The delicate internal workings of the living cell forming the lining of the lungs or internal organs, most certainly will be changed (mutated) or killed outright by the energetic alpha particle. The number of lung cancer cases among uranium miners from inhaled and ingested alpha sources is much higher than those of the public at large. Radon, the gas produced by the decay of radium-226, also emits alpha particles, which poses a hazard to lungs and airways when inhaled. Homes built in areas with high ground radioactivity should be tested for radon buildup in enclosed basement spaces.
BETA PARTICLES
Beta rays are much lighter energy particles. The beta particle is an energetic electron given off by the nucleus of unstable isotopes to restore an energy balance. They leave the nucleus at a speed of 270,000 kilometres per second. They can be stopped, for instance, by an aluminium sheet a few millimetres thick or by 3 metres of air. The RS-500 can detect most energetic beta particles through the case. Weaker beta particles can be detected through the tube window. Although the beta particle is around 8000 times smaller than the alpha particle, it is capable of penetrating much deeper into living matter. Each encounter with a living cell, and there may be many before the beta energy is dissipated, is likely to dam age some of the chemical links between the living molecules of the cell or cause some permanent genetic change in the cell nucleus. If the damage occurs within the generative cells of the ovaries or testes, the damage may be passed to new generations. The normal background radiation level must contribute to the mutation of the gene pool. Most mutations are undesirable with a very few leading to "improvements". Any increase in the background level of radiation should be considered harmful.
GAMMA RAYS
The next "particle" is the very high energy "X-ray" called the gamma ray. It is an energetic photon or light wave in the same electromagnetic family as light and x-rays, but is much more energetic and harmful. It is capable of damaging living cells as it slows down by transferring its energy to surrounding cell components. The RS-500 detects energetic gamma rays through the case walls. Gamma ray sources are used to find flaws in pipes and vessels and to check the integrity of welds in steel.
TERMS AND DEFINITIONS
Atomic number - The number of protons in the nucleus of the atom. Since the protons are positively charged, enough negatively charged electrons are collected around the nucleus to neutralize or charge balance the atom. These protons and electrons give the atom its unique chemical nature.
Atomic mass - The sum of the weights of both the neutrons and the protons in the atom.
Electron - A small negatively charged particle that surrounds the nucleus with a mass about 1/1800 that of the proton . Beta particles are energetic electrons ejected from a radioactive nucleus.
Element - the most basic physical substance composed of all the same type of atoms. Each atom will have the same number of protons. The number of neutrons can differ.
Isotope - Atoms with the same number of protons, but differing in the number of neutrons present in the nucleus. Most elements have more than one isotope.
Neutron - An electrically neutral particle found in the nucleus with a mass almost that of the proton. In the fission process, neutrons are liberated.
Nucleus - The densely packed kernel of the atom containing protons and neutrons. The diameter of the nucleus is 100,000 to 200,000 smaller than the whole atom.
Photon - The smallest unit of light. The photon is often described as a electromagnetic wave or wave packet. Light photons from red to blue in the visible spectrum have increasing energy. X-rays and gamma rays are energetic photons with thousands to millions of times the energy of light photons.
Proton - An electrically positive particle found in the nucleus of the atom. Each proton is balanced by the charge of an electron surrounding the nucleus. The electrically neutral atom has the same number of negative electrons as positive protons.
UNITS OF RADIATION MEASUREMENT
Roentgen (R) The roentgen is a unit used to measure a quantity called exposure. The roentgen measures the energy produced by gamma radiation in a cubic centimeter of air. This can only be used to describe an amount of gamma and X-rays, and only in air. One roentgen is equal to depositing in dry air enough energy to cause 2.58E-4 coulombs per kg. It is a measure of the ionizations of the molecules in a mass of air. The main advantage of this unit is that it is easy to measure directly, but it is limited because it is only for deposition in air, and only for gamma and x rays.
Rad (Radiation Absorbed Dose) Different materials that receive the same exposure may not absorb the same amount of energy. The rad is a unit used to measure a quantity called absorbed dose. This translates to the amount of energy actually absorbed in some material, and is used for any type of radiation and any material. One rad is defined as the absorption of 100 ergs per gram of material. One roentgen of gamma radiation exposure results in about one rad of absorbed dose. The unit rad can be used for any type of radiation, but it does not describe the biological effects of the different radiations.
Rem (Roentgen Equivalent Man) The rem is a unit used to derive a quantity called equivalent dose. This relates the absorbed dose in human tissue to the effective biological damage of the radiation. Not all radiation has the same biological effect, even for the same amount of absorbed dose. Equivalent dose is often expressed in terms of thousandths of a rem, or mrem. To determine equivalent dose (rem), you multiply absorbed dose (rad) by a quality factor (Q) that is unique to the type of incident radiation. For gamma rays and beta particles, 1 rad of exposure results in 1 rem of dose.
Curie (Ci) - 1Ci = 37 billion cps The curie is a unit used to measure a radioactivity. One curie is the number of particles per second from 1 gram of Radium = 3.7 x 10 E10 counts/second = 37 billion cps. = 37 billion Becquerel. Often radioactivity is expressed in smaller units like: thousandths (mCi), one millionths (uCi) or even billionths (nCi) of a curie. The relationship between becquerels and curies is: 3.7 x 1010 Bq in one curie.
1 microcurie = 1 uCi = 37,000 Bq = 37,000 cps.
1 microcurie = 2.22 x 10E6 disintegrations / minute = 2,220,000 cpm.
1 nanocurie = 1 billionth of a curie = 2,220 disintegrations / minute.
1 picocurie = 2.2 disintegrations / min.
Common Units - SI - International Standard
Note: These are the common units used throughout the world in health physics.
Gray (Gy) - Gray (Gy) = 1 Joule/kg. The gray is a unit used to measure a quantity called absorbed dose. This relates to the amount of energy actually absorbed in some material, and is used for any type of radiation and any material. One gray is equal to one joule of energy deposited in one kg of a material. The unit gray can be used for any type of radiation, but it does not't describe the biological effects of the different radiations. Absorbed dose is often expressed in terms of hundredths of a gray, or centi-grays. One gray is equivalent to 100 rads.
Sievert (Sv) 1Sv = 1Gray x QF, where QF is a "quality factor" based on the type of particle. The sievert is a unit used to derive a quantity called equivalent dose. This relates the absorbed dose in human tissue to the effective biological damage of the radiation. Not all radiation has the same biological effect, even for the same amount of absorbed dose. Equivalent dose is often expressed in terms of millionths of a sievert, or micro-sievert. To determine equivalent dose (Sv), you multiply absorbed dose (Gy) by a quality factor (Q) that is unique to the type of incident radiation. One sievert is equivalent to 100 rem. For electrons, positrons, and xrays = 1 QF = 3 to 10 for neutrons, protons dependent upon the energy transferred by these heavier particles. QF = 20 for alpha particles and fission fragments.
Becquerel (Bq) - 1Bq = 1 count per second = 1 event per second. The Becquerel is a unit used to measure a radioactivity. One Becquerel is that quantity of a radioactive material that will have 1 transformations in one second. Often radioactivity is expressed in larger units like: thousands (kBq), one millions (MBq) or even billions (GBq) of a becquerels. As a result of having one Becquerel being equal to one transformation per second, there are 3.7 x 1010 Bq in one curie.
Converting older units:
1 rad = 1 centigray = 10 milligrays ( 1 rad = 1cGy = 10 mGy )
1 rem = 1 centisievert = 10 millisieverts ( 1 rem = 1cSv = 10 mSv )
1 mrad = 10 uGy
Nominal background radiation absorbed dose of 100 mrad/year = 1 mGy/yr.
Nominal background radiation dose biological equivalent of 100mrem/year = 1mSv/yr.
Occupational whole body limit is 5 rem/yr = 50 mSv/yr. ( Recently proposed that levels be reduced to 2 rem/yr.)
2.5 mrem/hr or 25 uSv/hr is maximum average working level in industry.
Exposure rate from Naturally Occurring Radioactive Material (NORM) ; an empirically derived conversion factor for Ra-226 decay series: 1.82 microR/ hour = 1 picoCurie/gram.
![]()
Our new RS-500 radioactivity detector and meter measures nuclear radiation levels from the lowest background levels up to 999 mR/hr (10,000.00 ÁSv/h) (a level that can be reached only in a major nucelar accident or after the explosion of a nuclear weapon). This is 20 times more than ordinary radiation detection devices.
When set on, the alert will beep at a radioactivity level of 1mR/hr (standard nuclear industry radiation alert level).
The RS-500 radioactivity detector detects and measures Alpha, Beta, Gamma and X-Rays (A-B-G-X) radiation. Its digital display is easy to read and does not require switching between scales.
The RS-500 radioactivity detector is as portable as a small cell phone. It is designed for professional and personal use.
Because nuclear emergencies (nucelar terrorist attack, nuclear weapon, radioactive contamination, nukelar accident, etc...) may produce high to very high levels of radiation, the RS-500 high range makes it the best choice for Police departments, security and military personnel, as well as for individual and family safety. Other devices generally saturate before radiation levels become really dangerous and can measure only "safe" radiation levels. They become useless when they are most needed!
We recommend you keep your radiation detector at all time inside the vehicle you would use in case of a radioactivity emergency. This may save precious time should a major radioactive threat occur .